APPROVED USE & SAFETY INFO
APPROVED USE
IMPORTANT SAFETY INFORMATION
APPROVED USE
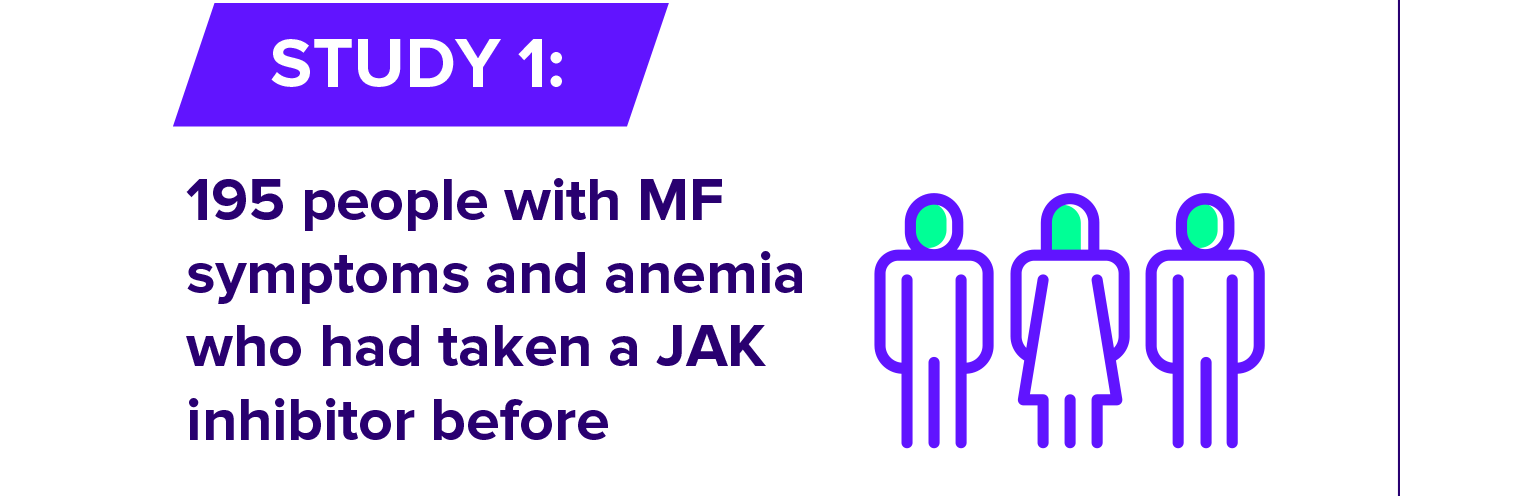
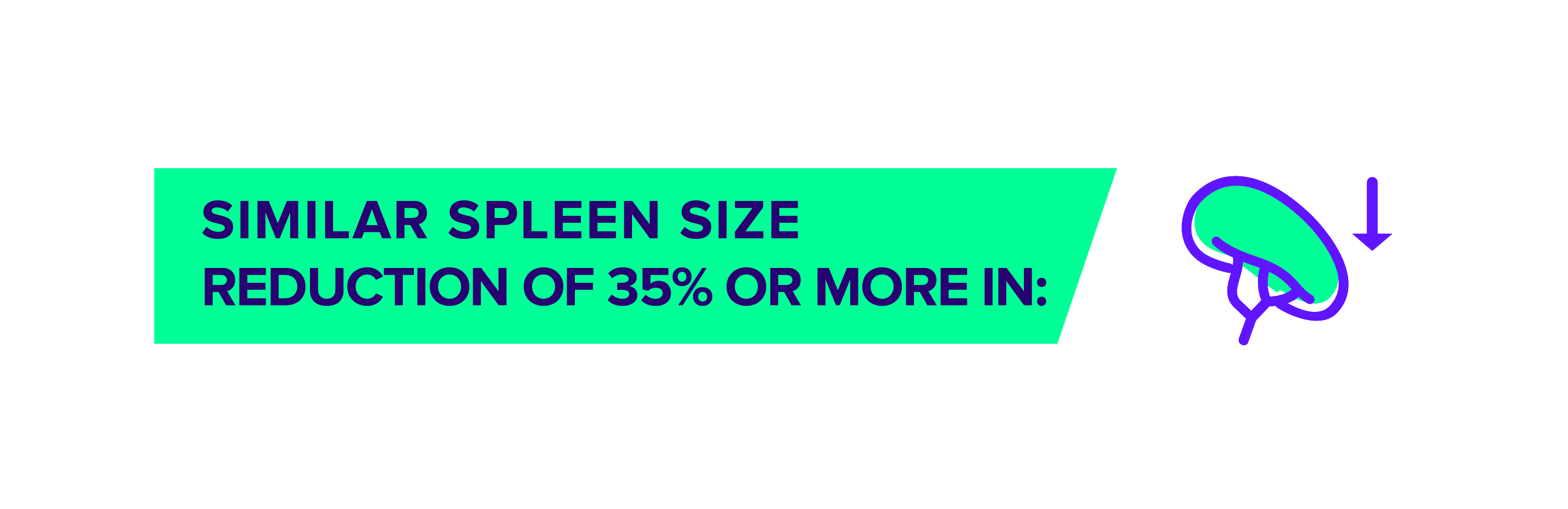
OJJAARA is a prescription medicine used to treat adults with certain types of myelofibrosis (MF) who have anemia. It is not known if OJJAARA is safe and effective in children.
IMPORTANT SAFETY INFORMATION
OJJAARA may cause serious side effects, including:
- Risk of Infections. People who take OJJAARA may develop serious infections that can lead to death, such as bacterial and viral infections, including COVID-19. If you have an active infection, your healthcare provider should not start treatment with OJJAARA until your infection is gone. If you have had hepatitis B for a long time (chronic), OJJAARA may cause your hepatitis B to become active again, and your healthcare provider will check your blood for active hepatitis B before starting treatment. Your healthcare provider will monitor you and treat you for any infections that you get during treatment with OJJAARA. Tell your healthcare provider right away if you develop any of the following symptoms of infection:
- fever
- chills
- cough
- breathing problems
- diarrhea
- vomiting
- pain or burning feeling when passing urine
- Low platelet and white blood cell counts. OJJAARA may cause new or worsening low platelet and white blood cell counts. Low platelet counts may increase your risk for bleeding and low white blood cell counts may increase your risk for infection. Your healthcare provider will do blood tests to check your blood counts before you start taking OJJAARA and during treatment. Tell your healthcare provider right away if you have any signs of bleeding during treatment with OJJAARA, including:
- unusual bleeding
- black or tarry stools
- bruising
- Liver problems. OJJAARA may cause new or worsening increased liver enzymes and bilirubin in your blood. Your healthcare provider will check your liver enzymes before starting treatment, every month for the first 6 months of treatment, and then as needed during treatment with OJJAARA. Your healthcare provider may stop treatment with OJJAARA if your liver enzymes increase. Tell your healthcare provider if you develop any of the following signs or symptoms of liver problems:
- tiredness
- loss of appetite
- pain in your right upper stomach area (abdomen)
- dark urine
- yellowing of your skin or the white part of your eyes
- Severe skin reactions. Severe skin reactions that can be life-threatening have occurred with OJJAARA. Tell your healthcare provider or get medical help right away if you get any of the following signs or symptoms of severe skin reactions, with or without fever:
- rash that keeps getting worse
- severe rash
- reddened skin
- flu-like symptoms
- skin pain or burning
- blistering of the lips, eyes, or mouth
- blisters on the skin
- skin peeling
- Major cardiovascular events such as heart attack, stroke, and death. Major cardiac events have happened, especially in people with cardiac risk factors and who are current or past smokers, taking another Janus kinase (JAK) inhibitor to treat rheumatoid arthritis. OJJAARA is in the JAK family of medicines. Get emergency help right away if you have any symptoms of a heart attack or stroke while taking OJJAARA, including:
- discomfort in your chest that lasts for more than a few minutes, or that goes away and comes back
- severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw
- pain or discomfort in your arms, back, neck, jaw, or stomach
- shortness of breath with or without chest discomfort
- breaking out in a cold sweat
- nausea or vomiting
- feeling lightheaded
- weakness in one part or on one side of your body
- slurred speech
- Blood clots. Blood clots in the veins of the legs (deep vein thrombosis, DVT) or lungs (pulmonary embolism, PE) have happened in some people taking another JAK inhibitor to treat rheumatoid arthritis, and may be life-threatening. Tell your healthcare provider if you have had blood clots in the veins of your legs or lungs in the past. Tell your healthcare provider right away if you have any signs and symptoms of blood clots during treatment with OJJAARA, including:
- swelling, pain, or tenderness in one or both legs
- sudden, unexplained chest pain
- shortness of breath or difficulty breathing
- New cancers. New cancers, including lymphoma and other cancers, except non-melanoma skin cancer, have happened in some people taking another JAK inhibitor to treat rheumatoid arthritis. The risk of new cancers is further increased in people who smoke or who smoked in the past.
The most common side effects of OJJAARA include:
- low platelet count
- bleeding
- bacterial infection
- tiredness
- dizziness
- diarrhea
- nausea
These are not all the possible side effects of OJJAARA. Call your doctor for medical advice about side effects.
Before taking OJJAARA, tell your healthcare provider about all your medical conditions, including if you:
- have an infection
- have or have had hepatitis B
- have or have had liver problems
- have had a heart attack, or have or have had other heart problems, or stroke
- have or have had a blood clot
- smoke or were a smoker in the past
- have or have had any other cancers
- are pregnant or plan to become pregnant. OJJAARA may harm your unborn baby.
Females who are able to become pregnant:- You should use effective birth control (contraception) during treatment and for 1 week after the last dose of OJJAARA.
- Tell your healthcare provider right away if you think you are pregnant or become pregnant during treatment with OJJAARA.
- are breastfeeding or plan to breastfeed. It is not known if OJJAARA passes into your breast milk. You should not breastfeed during treatment and for 1 week after the last dose of OJJAARA. Talk to your healthcare provider about the best way to feed your baby during this time.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking OJJAARA with certain other medicines may affect the amount of OJJAARA or the other medicines in your blood and may increase your risk of side effects.
Please see full Prescribing Information, including Patient Information for OJJAARA.